The Crucial Parts of a Neuronal Junction
The intricate dance of communication within the nervous system hinges on specialized contact points known as neuronal junctions, areas where nerve cells interact to transmit information. These junctions, fundamental to all brain functions, facilitate the transfer of electrical and chemical signals between neurons. At these sites, a symphony of molecular events unfolds, allowing the rapid and precise communication that underlies our thoughts, actions, and sensations. These highly specialized regions are not merely passive relay points; rather, they are active sites where signals are modulated, processed, and refined, thereby shaping the neural pathways and networks that define the brain’s capabilities. The efficiency and flexibility of neural communication are entirely dependent on the structural and functional integrity of these neuronal junctions. Understanding the processes at these junctions is critical to understanding the workings of the nervous system. The study of these structures forms a cornerstone of neuroscience research, allowing us to explore both normal brain function and the pathological processes that occur in neurological diseases. Examining the details of these junctions allows researchers to investigate the various elements that participate in the transmission process and explore how the structural organization contributes to the functional performance of the brain.
The process of neural signaling at these key points involves a precise sequence of events, starting with the arrival of an electrical impulse at the pre-synaptic terminal of a neuron. This electrical signal triggers a series of complex biochemical events that culminates in the release of chemical messengers known as neurotransmitters into a narrow space between the two interacting cells. These chemical messengers then travel across this space to influence the receiving cell. The receiving, or post-synaptic cell, has specialized receptor proteins that can bind to these released chemical signals. This interaction triggers further changes within the receiving cell, allowing the information to be passed along. The entire process, while appearing as a simple transfer of information, involves a series of carefully orchestrated molecular interactions within the neuronal junction. While we are not specifically detailing every single component here, it is crucial to note that each component, such as the vesicles, receptors, and specialized proteins, contributes to the fine-tuned and precise communication that is vital to neural function. Understanding the basic principle of neuronal communication begins with appreciating the role of these junctions.
The term “neuronal junction” encompasses a broad range of structures and processes, but it is important to appreciate that when discussing them in more detail, one is essentially investigating the various synapse components. These synapse components all participate in the process of signal transmission. The mechanisms and elements that contribute to signal transduction at these sites are crucial to comprehending neural connectivity and the brain’s operations. Without these functional units, the nervous system would not be able to process information or adapt to its environment. These interactions define all brain processes, from basic reflex actions to complex cognitive functions, highlighting the absolute importance of the neuronal junction.
How to Identify Key Structures at the Synapse
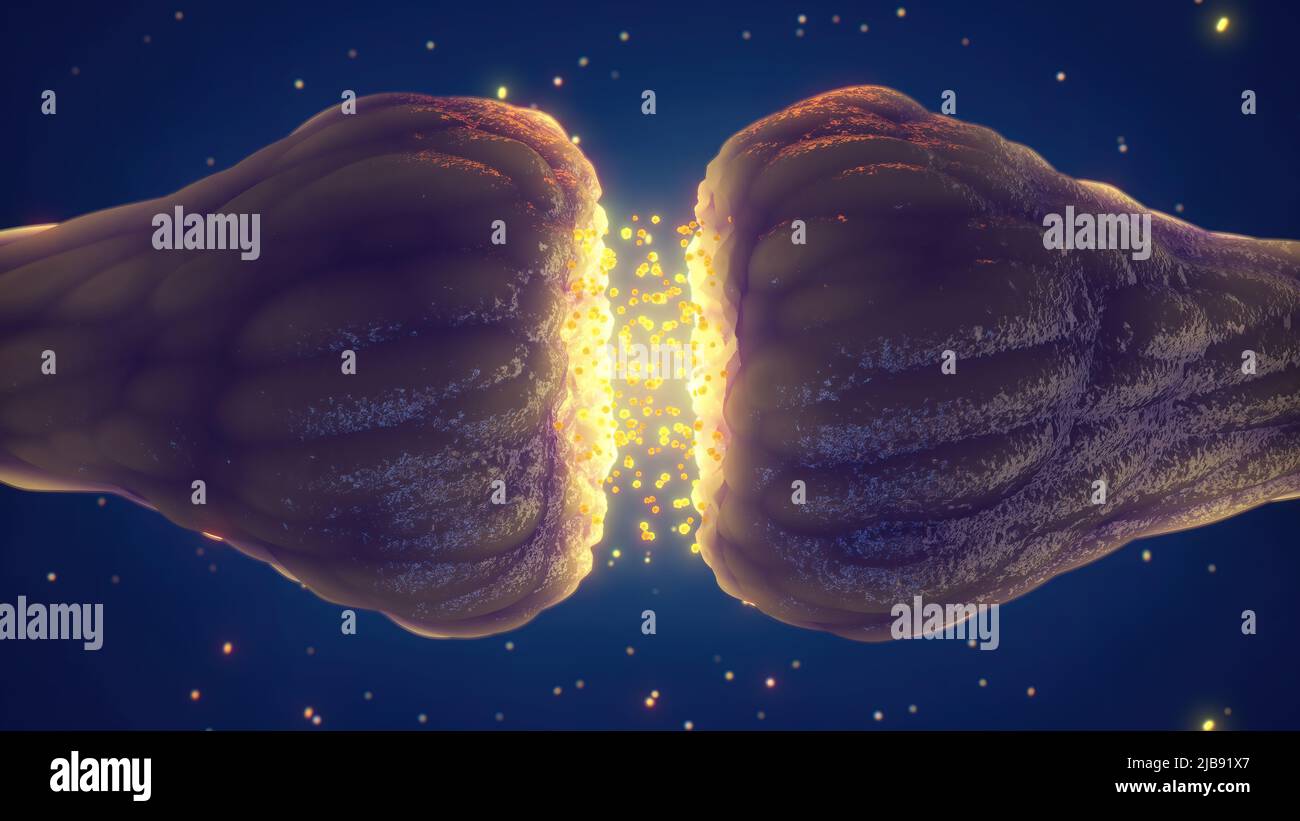
Examining a neuronal junction, often referred to as a synapse, requires a keen understanding of its structural organization. Whether through the lens of a powerful microscope or by studying detailed diagrams, one can discern the critical elements that facilitate neural communication. The process begins with distinguishing between two key sides: the presynaptic and the postsynaptic terminals. The presynaptic terminal, usually belonging to the neuron sending the signal, is the site where the neurotransmitters are prepared for release. It is characterized by the presence of various specialized structures vital for this function. On the other hand, the postsynaptic terminal, found on the neuron receiving the message, is designed to detect and respond to the released neurotransmitters. This is accomplished through highly specialized receptor proteins embedded in its membrane. Understanding how to differentiate these two terminals, and their respective functions, is crucial for appreciating the intricacies of neural communication and how various synapse components interact.
The examination of a synapse involves scrutinizing these pre- and postsynaptic structures as well as the intervening space. Diagrams often illustrate the presynaptic terminal as an area packed with small vesicles, which are the storage compartments for neurotransmitters, while the postsynaptic terminal typically features a dense region, known as the postsynaptic density, where receptors are concentrated. The synaptic cleft, a narrow gap between the two terminals, is also an important area of study, since it’s the medium through which the neurotransmitters pass, highlighting its crucial role in neural communication. When using microscopy techniques, careful staining protocols can be used to enhance the visibility of these synapse components, allowing researchers to observe the detailed architecture and arrangement within a synaptic junction, and to differentiate between the distinct zones of a neuron connection. Observing in detail the different synapse components is key to understand their specific functions.
The Presynaptic Terminal: Packaging and Release
The presynaptic terminal, a critical element of the neuronal junction, is a specialized area within the neuron responsible for initiating communication with other neurons. Its structure is intricately designed to facilitate the packaging and release of neurotransmitters. Key components within the presynaptic terminal include synaptic vesicles, small membrane-bound sacs that serve as storage units for neurotransmitters. These vesicles are not randomly dispersed but are actively loaded with neurotransmitters via specialized transporter proteins. This intricate process ensures that a readily available supply of neurotransmitters is always on hand when needed. Another critical structure is the active zone, a specialized area of the presynaptic membrane rich in proteins involved in vesicle docking and fusion. This is where the actual release of neurotransmitters into the synaptic cleft occurs. Among the most important proteins at the presynaptic terminal are the SNARE proteins, a complex set of proteins that facilitate the fusion of synaptic vesicles with the presynaptic membrane. The precision with which these proteins operate ensures the quick and efficient release of neurotransmitters upon arrival of an action potential. These physical structures, which are critical synapse components, enable this efficient signaling to happen.
The functionality of the presynaptic terminal is dependent on a harmonious orchestration of its structures. The process of neurotransmitter release is initiated by an action potential arriving at the presynaptic terminal, which triggers an influx of calcium ions. This calcium influx then interacts with the SNARE proteins to initiate the fusion of synaptic vesicles with the presynaptic membrane. This fusion process opens a pore through which the neurotransmitters are released into the synaptic cleft. The careful regulation of these events ensures the appropriate amount of neurotransmitter is released, preventing over- or under-stimulation of the postsynaptic neuron. Thus, the presynaptic terminal is not just a container but a dynamic site of active processing. Understanding the structural layout and the interaction of the synapse components within it is fundamental to understanding how the nervous system functions. The presynaptic terminal has different synapse components which are working together.
The presynaptic terminal also incorporates mechanisms for recycling synaptic vesicles, ensuring that a continuous supply is readily available. Following the release of neurotransmitters, the membrane of the synaptic vesicle is retrieved back into the presynaptic terminal by a process called endocytosis and is re-filled with neurotransmitters. This efficient recycling mechanism contributes to sustained synaptic communication. The arrangement of synapse components in the presynaptic terminal enables the process of neurotransmitter packaging, release and retrieval to be efficient and timely. This intricate system provides the structural basis for neural transmission and contributes to the complexity and adaptability of the nervous system. Without the precise function of these structural elements of the presynaptic terminal, communication between neurons would be greatly compromised.
The Synaptic Cleft: The Gap Between Neurons
The synaptic cleft represents a critical, albeit tiny, space separating the presynaptic and postsynaptic terminals. This intercellular gap, typically measuring around 20-40 nanometers, is not merely an empty void; rather, it’s a dynamic environment filled with extracellular fluid and various molecules that play crucial roles in regulating neurotransmission. The precise size of the synaptic cleft is vital, as it influences the time it takes for neurotransmitters to diffuse across this space and reach the postsynaptic receptors. This diffusion time directly impacts the speed and efficacy of synaptic communication. Variations in the cleft’s width can be indicative of synaptic plasticity, a fundamental mechanism for learning and memory. The composition of the synaptic cleft’s environment also matters. For instance, various enzymes are present that can degrade neurotransmitters, effectively terminating the signal and preventing overstimulation of the postsynaptic neuron. Additionally, adhesion molecules within the cleft contribute to the structural integrity of the synapse, ensuring that the pre- and postsynaptic terminals remain properly aligned.
The extracellular fluid found within the synaptic cleft contains a complex mixture of ions, proteins, and other bioactive molecules that can modulate neurotransmission. Some of these molecules may directly interact with neurotransmitters or receptors, influencing the strength and duration of the synaptic signal. The carefully maintained environment of the synaptic cleft is crucial for maintaining a stable and reliable signaling pathway. Disruptions to the cleft, whether in its size or molecular composition, can lead to significant impairments in neural function. Therefore, understanding the interplay of various synapse components within this tiny space is paramount to understanding how neural signals are transmitted and processed in the brain. Considering this, a clear comprehension of these minute, but essential structures that are critical to the operation of the brain can enhance a better understanding of the overall mechanisms of the neural system.
The Postsynaptic Terminal: Receiving the Message
The postsynaptic terminal, the receiving end of the neuronal junction, is specifically designed to detect and respond to neurotransmitter signals released from the presynaptic terminal. This region is characterized by several crucial synapse components that facilitate the conversion of chemical signals into electrical responses. Central to this process are receptors, specialized protein molecules embedded in the postsynaptic membrane. These receptors are the first point of contact for neurotransmitters, acting as molecular locks that bind to specific neurotransmitter keys. There are two main classes of receptors: ionotropic receptors, which are ligand-gated ion channels, and metabotropic receptors, which initiate intracellular signaling cascades. Ionotropic receptors, upon neurotransmitter binding, rapidly open to allow specific ions to flow across the membrane, directly altering the postsynaptic neuron’s membrane potential. This mechanism facilitates a fast and direct response to incoming signals. In contrast, metabotropic receptors activate intracellular signaling pathways, which may indirectly influence ion channels or alter other cellular processes. This action tends to be slower and longer-lasting, allowing for a diverse array of cellular responses. The postsynaptic density (PSD), a protein-rich region directly beneath the postsynaptic membrane, plays a vital role in organizing and regulating the activity of these receptors. It also contains numerous other proteins that are essential for signal transduction and synaptic plasticity, the ability of the synapse to change in strength and efficacy over time.
The physical structure of the postsynaptic terminal is critical for its functional role. The postsynaptic membrane is often folded or contains specialized structures such as dendritic spines, which provide a large surface area for the reception of neurotransmitter signals and compartmentalize the effects of synaptic inputs. The density of receptors and other synapse components varies depending on the neuron type, brain region, and the type of neurotransmitter involved. Furthermore, the precise arrangement of receptors within the postsynaptic membrane, along with the PSD’s protein composition, contributes to the specificity and efficiency of synaptic transmission. Dysfunction in any of these structures or molecules can lead to altered neuronal signaling and contribute to neurological disorders. The complex interactions of these various elements in the postsynaptic terminal are essential for the integration of signals, processing of information, and overall functioning of the nervous system. Thus, a detailed understanding of these components is fundamental to comprehending how neurons communicate and how neural circuits operate.
Neurotransmitters: The Chemical Messengers
Neurotransmitters, crucial elements within neural communication, function as the primary chemical messengers facilitating signal transmission across neuronal junctions. These molecules are synthesized within the neuron, packaged into synaptic vesicles within the presynaptic terminal, and are released into the synaptic cleft in response to an action potential. This release process involves complex mechanisms, and the neurotransmitter then diffuses across the gap to interact with receptors on the postsynaptic membrane. The interaction is highly specific: neurotransmitters bind to receptors designed to recognize them. This binding event initiates a cascade of events, ultimately affecting the excitability of the postsynaptic neuron. This intricate dance between neurotransmitters and receptors at the neuronal junction is fundamental to all brain functions. Understanding these interactions is key to grasping the complete picture of how neural communication takes place, and how various synapse components work together to allow these exchanges.
Neurotransmitters come in a diverse array, each with distinct chemical structures and varying effects on the postsynaptic neuron. Broadly, they can be categorized as either excitatory or inhibitory. Excitatory neurotransmitters, such as glutamate, increase the likelihood of the postsynaptic neuron firing an action potential. Conversely, inhibitory neurotransmitters, like GABA, decrease this probability. The balance between excitatory and inhibitory signals at synapses shapes overall neuronal activity. Other key neurotransmitters include dopamine, which plays a crucial role in reward and motor control, and serotonin, involved in mood regulation. Different types of neurons utilize different combinations of neurotransmitters, and these precise interactions, involving all synapse components, provide fine-grained control over the flow of information within neural circuits. Therefore, the type of neurotransmitter released, along with its specific postsynaptic receptor, determines the nature of the transmitted message. These different synapse components act together, allowing for the great versatility and intricacy of brain activity.
The Importance of Astrocytes in Synaptic Function
Beyond the neuronal elements, astrocytes, a type of glial cell, play a crucial role in modulating synapse structure and function, adding another layer of complexity to neural communication. These star-shaped cells are not passive bystanders; they actively participate in shaping the environment of the neuronal junction and influencing the transmission of signals. Astrocytes extend numerous processes that envelop both the pre- and postsynaptic terminals, creating a tripartite structure where they can dynamically interact with the neuronal components. Their involvement is multifaceted, contributing significantly to the fine-tuning of synaptic activity. One of their key functions is the uptake and recycling of neurotransmitters. After a neurotransmitter has been released into the synaptic cleft and has exerted its effect on the postsynaptic neuron, astrocytes quickly remove the excess through specialized transporter proteins. This prevents overstimulation of the postsynaptic neuron and allows for a more controlled and precise signaling. For instance, astrocytes are particularly adept at taking up glutamate, the main excitatory neurotransmitter, converting it into glutamine, and releasing it back to the neurons, thus replenishing the neurotransmitter supply. This recycling is critical for maintaining a constant signal flow in neuronal circuits.
Furthermore, astrocytes contribute to the regulation of the ionic environment surrounding the synapse, particularly potassium (K+) homeostasis. During periods of intense neuronal activity, K+ concentrations can increase in the extracellular space, potentially disrupting the delicate electrical balance needed for efficient neuronal firing. Astrocytes, with their ability to take up excess K+, are essential in preventing such disruptions, which makes them an important part of the synapse components and its normal function. These cells are also known to release various substances known as gliotransmitters, including glutamate, ATP, and D-serine, which can directly influence synaptic transmission and plasticity. Depending on the specific context and the receptors they activate on pre- and postsynaptic elements, they can either enhance or inhibit neuronal communication, thus providing another level of control over the nervous system. Importantly, the dynamic interactions between astrocytes and neurons are not fixed but rather can change in response to neuronal activity and other conditions. Such plasticity underscores the dynamic nature of brain function, and highlights how the different synapse components are not static elements but active participants in the flow of information. The functional role of these cells highlights that neural communication cannot be fully understood without considering all the elements that influence it.
Putting It All Together: An Integrated View of Neural Communication
The intricate process of neural communication hinges on the precise orchestration of various structural and functional elements at the neuronal junction. From the initial packaging of neurotransmitters within the presynaptic terminal’s vesicles, to the release of these chemical messengers into the synaptic cleft, every step is vital for accurate signal transmission. The presynaptic terminal, characterized by its active zones and a complex of SNARE proteins, ensures that neurotransmitters are released at the appropriate time and in the correct amount. The synaptic cleft, a narrow gap between the pre- and postsynaptic terminals, facilitates diffusion of the neurotransmitter, playing a key role in the speed and accuracy of transmission. The postsynaptic terminal, equipped with a rich array of receptors – both ionotropic and metabotropic – is specialized to recognize these neurotransmitters and translate the chemical signal into an electrical or metabolic response within the receiving neuron. The postsynaptic density, a protein-rich structure located beneath the postsynaptic membrane, plays a crucial role in signal transduction, amplifying and modulating the incoming information. Thus, the careful arrangement and coordinated action of these neuronal junction structures are paramount for efficient communication within the nervous system. The synapse components together create a dynamic system that is key to all neural activity.
The overall efficacy of this system is further influenced by the presence and activity of astrocytes. These glial cells, once viewed solely as supporting cells, are now known to actively participate in synaptic function. They actively regulate neurotransmitter concentrations within the cleft by performing neurotransmitter uptake and recycling. This regulation process is vital for preventing overstimulation of the postsynaptic terminal, ensuring the signal remains clear and the circuit responsive. In addition, astrocytes provide structural support and metabolic assistance to neurons, contributing to the overall health and stability of the neural network. This highlights the point that the functionality of the neuronal junction and synapse components depends not only on the neuron itself but is modulated by these auxiliary cells. Therefore, the combined effect of the presynaptic structures, synaptic cleft, postsynaptic elements, and the supportive influence of astrocytes are necessary for effective information processing in the brain. These coordinated actions are essential for all neuronal communication and its associated brain activity.
The interaction between these various synapse components showcases how the nervous system manages and processes information. The process begins with the packaging of neurotransmitters in vesicles within the presynaptic neuron, travels through the synapse in the synaptic cleft to reach the receptors in the postsynaptic terminal, ultimately affecting the target cell’s membrane potential or cellular activity. It is a precise and tightly regulated process, where each element contributes to the accuracy and reliability of neural signaling. The dynamic interchange of chemical signals at the neuronal junction, modulated by all elements working in harmony, underscores the complexity and efficiency of neural networks. The interplay between all the synapse components creates a powerful system that underlies higher cognitive functions, behaviors, and all other activities controlled by the nervous system.